Amino acids (AAs), ubiquitous biological molecules, have long been known to stabilize biological systems. However, the underlying mechanism of this stabilization remained unclear, with hypotheses ranging from water structuring to specific protein interactions. This study reveals a fundamental, previously unknown colloidal property of AAs: their ability to broadly stabilize nanoscale colloidal dispersions, regardless of their biological origin.
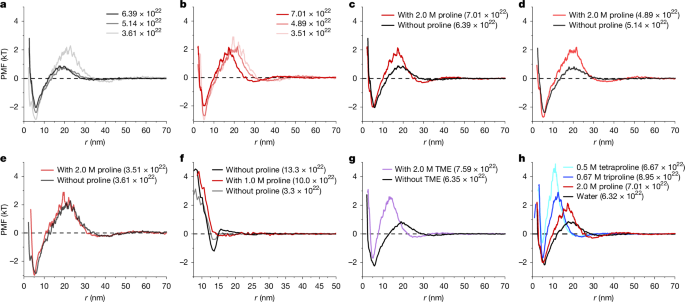
Researchers investigated the impact of AAs on the stability of various dispersions, including proteins (lysozyme, bovine serum albumin, apoferritin, FUS low-complexity domain), plasmid DNA, and non-biological gold nanoparticles. Using techniques such as analytical ultracentrifugation (AUC), self-interaction chromatography (SIC), and cryogenic electron microscopy (cryo-EM), they measured the second osmotic virial coefficient (B22) and the potential of mean force (PMF). A consistent increase in B22 across all systems upon AA addition indicated enhanced colloidal stability, achieved even at low AA concentrations (as low as 10 mM) and remarkably low protein-to-AA ratios (1:7).
The observed stabilization effect isn't attributable to protein-specific mechanisms like misfolding prevention. The researchers demonstrated this by observing similar stabilization of gold nanoparticles, which lack the conformational flexibility of proteins. Furthermore, the addition of 1 M proline to insulin significantly doubled its bioavailability in vivo, highlighting the practical implications of this discovery.
A novel theoretical framework was developed to explain the experimental observations. The model posits that weak AA-colloid interactions lead to a time-averaged screening of attractive colloid-colloid interactions. The theory successfully predicted several key experimental findings, including the charge-specificity of AA stabilization (charged AAs are effective only against oppositely charged proteins), the effectiveness of short peptides, and the general applicability of this stabilizing effect to small molecules beyond AAs. The theoretical predictions showed excellent quantitative agreement with experimental data, further validating the proposed mechanism.
The study provides substantial evidence for the generic colloidal stabilizing effect of AAs and short peptides, suggesting a far-reaching impact on biological processes and pharmaceutical formulation development. The ability of AAs to modulate protein-protein interactions, as demonstrated by changes in phase diagrams and stress granule formation in HeLa cells, underlines their significance in cellular processes. The research also highlights the potential for improving therapeutic protein formulations, as exemplified by the enhanced bioavailability of insulin achieved with a simple proline-based formulation. These findings suggest a significant reevaluation of the role of small molecules like AAs in both biological systems and pharmaceutical design is warranted.
---
Originally published at: https://www.nature.com/articles/s41586-025-09506-w
