Repetitive head impacts (RHIs) from contact sports pose a significant risk for chronic traumatic encephalopathy (CTE), a debilitating neurodegenerative disease. Currently, CTE is diagnosable only post-mortem, and the mechanisms triggering its hallmark hyperphosphorylated tau (p-tau) deposition remain unclear. This study investigated the early cellular responses to RHIs in young athletes (under 51 years old), predominantly American football players, to understand the disease's early pathogenesis and identify potential therapeutic targets.
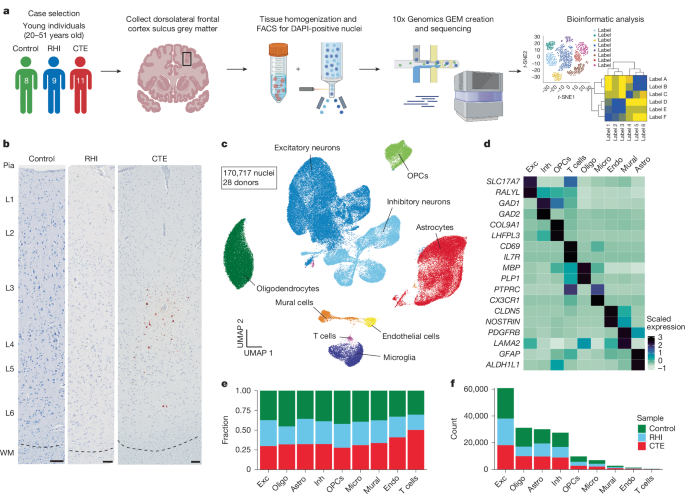
Using single-nucleus RNA sequencing (snRNA-seq) on brain tissue from control individuals, RHI-exposed individuals without CTE, and individuals with early-stage CTE, researchers identified a complex multicellular response preceding p-tau pathology. This response correlated with the duration of RHI exposure. Key findings included the presence of SPP1-expressing inflammatory microglia, angiogenic and inflamed endothelial cells, astrocytosis, and altered synaptic gene expression in RHI-exposed individuals.
Significantly, the study revealed a substantial loss of cortical sulcus layer 2/3 neurons independent of p-tau pathology. This neuronal loss was directly correlated with the number of years of RHI exposure. Further analysis using multiplex in situ hybridization and immunohistochemistry validated these snRNA-seq findings. The observed neuronal loss occurred specifically in the sulcus, the area most susceptible to mechanical forces during head trauma.
Microglia, the brain's resident immune cells, played a central role. Researchers identified distinct microglial subtypes, some displaying homeostatic functions and others exhibiting inflammatory phenotypes (RHIM1-3). The proportion of homeostatic microglia decreased with increasing years of RHI exposure, while inflammatory microglia increased. These inflammatory microglia, particularly RHIM2 and RHIM3, expressed genes associated with immune signaling, hypoxia, and metabolic processes.
The study also examined the vascular response, finding that capillary endothelial cells exhibited upregulation of angiogenesis and inflammation-associated genes. The increased expression of collagen genes suggested a potential early fibrotic response. Notably, TGFβ1 emerged as a potential mediator of microglia-endothelial cell crosstalk, potentially driving the observed inflammatory processes and neuronal loss.
The findings provide strong evidence that RHIs alone can induce lasting cellular alterations that precede p-tau deposition. The significant neuronal loss and the specific inflammatory microglial and endothelial responses identified offer potential targets for diagnostic and therapeutic interventions. Further research is needed to fully elucidate the mechanisms driving these early cellular changes and to develop effective strategies for preventing or treating CTE in young athletes.
---
Originally published at: https://www.nature.com/articles/s41586-025-09534-6
